Pipeline
| Discovery | Preclinical | Phase I | Phase II | Phase III | Launch | |
|---|---|---|---|---|---|---|
| IRX-001 (NDMC) Indication: neuropathic pain |
|
|||||
| IRX-002 Indication: undisclosed |
|
|||||
Phase I Clinical Trial
- Most analgesics used for neuropathic pain impair cognitive performance, while NDMC had similar effects on cognitive skills and sedation as compared to placebo.
- This anti-hyperalgesic activity in chronic pain also aims to enhance the competence of interprofessional healthcare providers involved in treating patients with chronic neuropathic pain by providing them with the safely necessary skills to improve patient care.
- Phase I clinical trial with UVB pain model in 32 healthy subjects testing IRX-001 (NDMC) 20/60 mg vs clonazepam 1,5 mg & placebo
|
% decrease from baseline
|
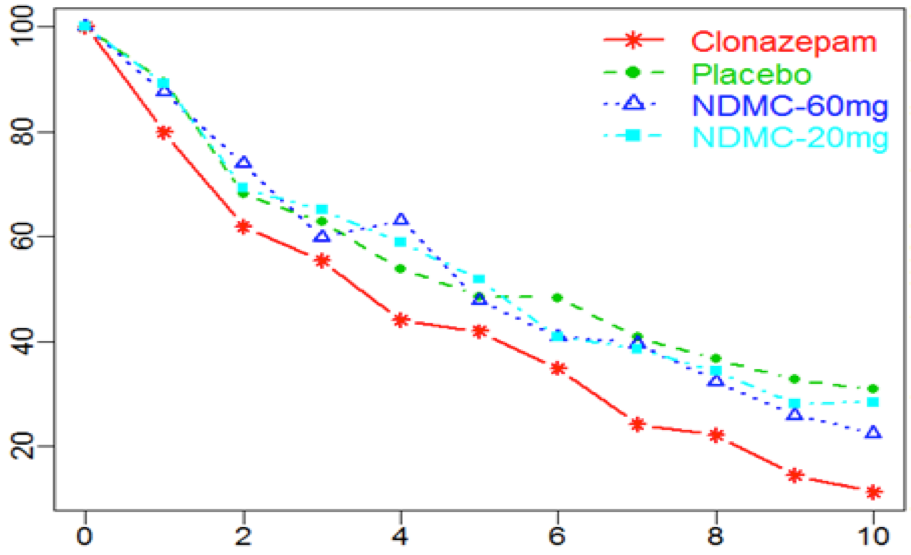
|
| time (h) |
Matthey et al. Eur J Pain. 2020
- Pharmacodynamic response at Cmax
- No sedation
Phase IIa Clinical Trial
- Phase IIa clinical trial with IRX-001 (NDMC) 40/60/120 mg vs placebo as add-on to standard of care in 29 non-responder or intolerant patients with chronic neuropathic pain
- Pain score measured on a VAS scale from 0 to 10
- Results: average improvement in pain score with IRX-001, compared to placebo, is of 0.5 point on the VAS scale, in the range of trial results obtained with neuropathic pain standard of care duloxetine and pregabaline (Besson et al. in preparation)
- Magnitude of pain relief increases with IRX-001 plasma concentrations
- Very well tolerated, no sedation, no serious adverse events related to IRX-001 during trial